How to build electronic formulas of chemical elements. Electronic configuration of an atom
Algorithm for compiling the electronic formula of an element:
1. Determine the number of electrons in an atom using the Periodic Table of Chemical Elements D.I. Mendeleev.
2. By the number of the period in which the element is located, determine the number of energy levels; the number of electrons in the last electronic level corresponds to the group number.
3. Divide the levels into sublevels and orbitals and fill them with electrons in accordance with the rules for filling orbitals:
It must be remembered that the first level has a maximum of 2 electrons. 1s2, on the second - a maximum of 8 (two s and six R: 2s 2 2p 6), on the third - a maximum of 18 (two s, six p, and ten d: 3s 2 3p 6 3d 10).
- Principal quantum number n should be minimal.
- Filled in first s- sublevel, then p-, d-b f- sublevels.
- Electrons fill orbitals in ascending order of orbital energy (Klechkovsky's rule).
- Within the sublevel, electrons first occupy free orbitals one at a time, and only after that they form pairs (Hund's rule).
- There cannot be more than two electrons in one orbital (Pauli principle).
Examples.
1. Compose the electronic formula of nitrogen. Nitrogen is number 7 on the periodic table.

2. Compose the electronic formula of argon. In the periodic table, argon is at number 18.
1s 2 2s 2 2p 6 3s 2 3p 6.

3. Compose the electronic formula of chromium. In the periodic table, chromium is number 24.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5

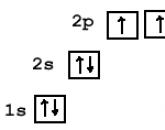
Energy diagram of zinc.
4. Compose the electronic formula of zinc. In the periodic table, zinc is number 30.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10
Note that part of the electronic formula, namely 1s 2 2s 2 2p 6 3s 2 3p 6 is the electronic formula of argon.
The electronic formula of zinc can be represented as.
In order to learn how to compose electron-graphic formulas, it is significant to realize the theory of the structure of the nuclear nucleus. The nucleus of an atom is made up of protons and neutrons. Electrons are located in electron orbitals around the nucleus of an atom.
You will need
- - pen;
- - note paper;
- - the periodic system of elements (Mendeleev's table).
Instruction
1. Electrons in an atom occupy vacant orbitals in a sequence called the energy scale: 1s/2s, 2p/3s, 3p/4s, 3d, 4p/5s, 4d, 5p/6s, 4d, 5d, 6p/7s, 5f, 6d, 7p . Two electrons with opposite spins - directions of rotation can be located on one orbital.
2. The design of electron shells is expressed with the support of graphic electronic formulas. Use a matrix to write a formula. One cell can contain one or two electrons with opposite spins. Electrons are represented by arrows. The matrix clearly shows that two electrons can be located in the s-orbital, 6 in the p-orbital, 10 in the d-orbital, and 14 in the f-orbital.

3. Consider the rule for compiling an electronic graphic formula using manganese as an example. Find manganese in the periodic table. Its serial number is 25, which means there are 25 electrons in the atom, this is an element of the fourth period.
4. Write down the serial number and symbol of the element next to the matrix. In accordance with the energy scale, fill in the 1s, 2s, 2p, 3s, 3p, 4s tiers step by step, entering two electrons per cell. You get 2+2+6+2+6+2=20 electrons. These tiers are completely filled.

5. You have five more electrons left and an empty 3d tier. Arrange the electrons in the cells of the d-sublevel, starting from the left. Place the electrons with identical spins in the cells first one by one. If all cells are filled, starting from the left, add a second electron with the opposite spin. Manganese has five d-electrons, located one at a time in the entire cell.

6. Electron graphic formulas clearly show the number of unpaired electrons that determine the valence.
When creating theoretical and factual works in mathematics, physics, chemistry, a student or schoolchild is faced with the need to insert special symbols and difficult formulas. Having the Word application from the Microsoft office suite, it is allowed to type an electronic formula every difficulty.

Instruction
1. Open the newest document in Microsoft Word. Give it a name and save it in the same folder where your work is, so that you don’t look for it in the future.
2. Go to the "Insert" tab. On the right, find the symbol ?, and next to it is the inscription "Formula". Click on the arrow. A window will appear where you can prefer a built-in formula, say a quadratic equation formula.
3. Click on the arrow and a variety of symbols will appear on the top panel that you may need when writing this particular formula. By changing it the way you want it, you can save it. From now on, it will drop out in the list of built-in formulas.
4. If you need to transfer the formula to text, the one that later needs to be placed on the site, then click on the energetic field with it with the right mouse button and select not the highly professional, but the linear method of writing. In particular, the formula of the same quadratic equation in this case will take the form: x=(-b±?(b^2-4ac))/2a.
5. Another option for writing an electronic formula in Word is through the constructor. Hold down the Alt and = keys at the same time. You will immediately have a field for writing a formula, and a constructor will open in the top panel. Here you can prefer all the signs that may be required to write an equation and solve any problem.
6. Some linear notation symbols may be obscure to a reader unfamiliar with computer symbols. In this case, it makes sense to save the most difficult formulas or equations in graphical form. To do this, open the easiest graphic editor Paint: "Start" - "Programs" - "Paint". After that, zoom in on the formula document so that it takes up every screen. This is necessary so that the saved image has the highest resolution. Press PrtScr on your keyboard, go to Paint and press Ctrl+V.
7. Trim off any excess. As a result, you will get a solid image with the necessary formula.
Related videos
Note!
Remember that chemistry is a science of exceptions. The atoms of the secondary subgroups of the Periodic system have an electron "breakthrough". For example, in chromium with atomic number 24, one of the electrons from the 4s-tier goes to the d-tier cell. Molybdenum, niobium, etc. have a similar result. In addition, there is a representation of the excited state of the atom, when paired electrons are unpaired and transferred to neighboring orbitals. Therefore, when compiling electronic graphic formulas for the elements of the fifth and subsequent periods of the secondary subgroup, refer to the reference book.
When writing electronic formulas of atoms of elements, energy levels are indicated (values of the main quantum number n in the form of numbers - 1, 2, 3, etc.), energy sublevels (values of the orbital quantum number l in the form of letters s, p, d, f) and the number at the top indicates the number of electrons in a given sublevel.
The first element in the D.I. Mendeleev is hydrogen, therefore, the charge of the nucleus of an atom H equal to 1, the atom has only one electron per s sublevel of the first level. Therefore, the electronic formula of the hydrogen atom is:
The second element is helium, there are two electrons in its atom, therefore the electronic formula of the helium atom is 2 Not 1s 2. The first period includes only two elements, since the first energy level is filled with electrons, which can only be occupied by 2 electrons.
The third element in order - lithium - is already in the second period, therefore, its second energy level begins to be filled with electrons (we talked about this above). The filling of the second level with electrons begins with s-sublevel, so the electronic formula of the lithium atom is 3 Li 1s 2 2s 1 . In the beryllium atom, filling with electrons is completed s- sublevels: 4 Ve 1s 2 2s 2 .
For subsequent elements of the 2nd period, the second energy level continues to be filled with electrons, only now it is filled with electrons R- sublevel: 5 IN 1s 2 2s 2 2R 1 ; 6 WITH 1s 2 2s 2 2R 2 … 10 Ne 1s 2 2s 2 2R 6 .
Neon atom completes filling with electrons R-sublevel, this element ends the second period, it has eight electrons, since s- And R-sublevels can contain only eight electrons.
The elements of the 3rd period have a similar sequence of filling the energy sublevels of the third level with electrons. The electronic formulas of atoms of some elements of this period are:
11 Na 1s 2 2s 2 2R 6 3s 1 ; 12 mg 1s 2 2s 2 2R 6 3s 2 ; 13 Al 1s 2 2s 2 2R 6 3s 2 3p 1 ;
14 Si 1s 2 2s 2 2R 6 3s 2 3p 2 ;…; 18 Ar 1s 2 2s 2 2R 6 3s 2 3p 6 .
The third period, like the second, ends with an element (argon), which completes its filling with electrons R–sublevel, although the third level includes three sublevels ( s, R, d). According to the above order of filling the energy sublevels in accordance with the rules of Klechkovsky, the energy of sublevel 3 d more sublevel 4 energy s, therefore, the potassium atom following the argon and the calcium atom following it is filled with electrons 3 s- sublevel of the fourth level:
19 TO 1s 2 2s 2 2R 6 3s 2 3p 6 4s 1 ; 20 Sa 1s 2 2s 2 2R 6 3s 2 3p 6 4s 2 .
Starting from the 21st element - scandium, in the atoms of the elements, sublevel 3 begins to fill with electrons d. The electronic formulas of the atoms of these elements are:
21 sc 1s 2 2s 2 2R 6 3s 2 3p 6 4s 2 3d 1 ; 22 Ti 1s 2 2s 2 2R 6 3s 2 3p 6 4s 2 3d 2 .
In the atoms of the 24th element (chromium) and the 29th element (copper), a phenomenon is observed called the “breakthrough” or “failure” of an electron: an electron from an external 4 s-sublevel "fails" by 3 d– sublevel, completing its filling by half (for chromium) or completely (for copper), which contributes to greater stability of the atom:
24 Cr 1s 2 2s 2 2R 6 3s 2 3p 6 4s 1 3d 5 (instead of ...4 s 2 3d 4) and
29 Cu 1s 2 2s 2 2R 6 3s 2 3p 6 4s 1 3d 10 (instead of ...4 s 2 3d 9).
Starting from the 31st element - gallium, the filling of the 4th level with electrons continues, now - R– sublevel:
31 Ga 1s 2 2s 2 2R 6 3s 2 3p 6 4s 2 3d 10 4p 1 …; 36 Kr 1s 2 2s 2 2R 6 3s 2 3p 6 4s 2 3d 10 4p 6 .
This element ends the fourth period, which already includes 18 elements.
A similar order of filling energy sublevels with electrons takes place in the atoms of elements of the 5th period. The first two (rubidium and strontium) are filled s- sublevel of the 5th level, the next ten elements (from yttrium to cadmium) are filled d– sublevel of the 4th level; six elements complete the period (from indium to xenon), in the atoms of which electrons are filled R-sublevel of the outer, fifth level. There are also 18 elements in a period.
For elements of the sixth period, this filling order is violated. At the beginning of the period, as usual, there are two elements, in the atoms of which is filled with electrons s-sublevel of the outer, sixth, level. At the next element - lanthanum - begins to fill with electrons d–sublevel of the previous level, i.e. 5 d. On this filling with electrons 5 d-sublevel stops and the next 14 elements - from cerium to lutetium - begin to fill f- sublevel of the 4th level. These elements are all included in one cell of the table, and below is an expanded series of these elements, called lanthanides.
Starting from the 72nd element - hafnium - to the 80th element - mercury, filling with electrons continues 5 d- sublevel, and the period ends, as usual, with six elements (from thallium to radon), in the atoms of which it is filled with electrons R-sublevel of the outer, sixth, level. This is the largest period, including 32 elements.
In the atoms of the elements of the seventh, incomplete, period, the same order of filling the sublevels is seen, as described above. We allow students to write electronic formulas of atoms of elements of the 5th - 7th periods, taking into account all that has been said above.
Note:in some textbooks, a different order of writing the electronic formulas of the atoms of the elements is allowed: not in the order in which they are filled, but in accordance with the number of electrons given in the table at each energy level. For example, the electronic formula of an arsenic atom may look like: As 1s 2 2s 2 2R 6 3s 2 3p 6 3d 10 4s 2 4p 3 .
An atom is an electrically neutral system consisting of a positively charged nucleus and negatively charged electrons. Electrons are located in the atom, forming energy levels and sublevels.
The electronic formula of an atom is the distribution of electrons in an atom over energy levels and sublevels in accordance with the principle of least energy (Klechkovsky), Pauli's principle, Hund's rule.
The state of an electron in an atom is described using a quantum mechanical model - an electron cloud, the density of the corresponding sections of which is proportional to the probability of finding an electron. Usually, the electron cloud is understood as the region of the nuclear space, which covers approximately 90% of the electron cloud. This region of space is also called an orbital.
Atomic orbitals form an energy sublevel. Orbitals and sublevels are assigned letter designations. Each sublevel has a certain number of atomic orbitals. If the atomic orbital is depicted as a magnetic-quantum cell, then the atomic orbitals located at sublevels can be represented as follows:
Each atomic orbital can contain no more than two electrons at the same time, differing in spin (Pauli principle). This difference is indicated by arrows ¯. Knowing that on s-sublevel one s-orbital, on R-sublevel three R-orbitals, on d-sublevel five d-orbitals, on f-sublevel seven f- orbitals, you can find the maximum number of electrons in each sublevel and level. Yes, on s-sublevel, starting from the first energy level, 2 electrons; on R-sublevel, starting from the second energy level, 6 electrons; on d-sublevel, starting from the third energy level, 10 electrons; on f-sublevel, starting from the fourth energy level, 14 electrons. Electrons on s-, p-, d-, f- sublevels are named respectively s-, p-, d-, f-electrons.
According to principle of least energy, the successive filling of energy sublevels with electrons occurs in such a way that each electron in an atom occupies a sublevel with the lowest energy corresponding to its strong bond with the nucleus. The change in the energy of sublevels can be represented as a Klechkovsky series or an energy scale:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p...
According to Hund's rule, each quantum cell (orbital) of the energy sublevel is first filled with single electrons with the same spin, and then with a second electron with the opposite spin. Two electrons with opposite spins in the same atomic orbital are called paired electrons. Single electrons are unpaired.
Example 1 Place 7 electrons on d-sublevel, taking into account the Hund's rule.
Solution.
On d sublevel - five atomic orbitals. The energy of orbitals that are at the same sublevel is the same. Then d sublevel can be represented as follows: d ![]() . After filling the atomic orbitals with electrons, taking into account the Hund's rule d-sublevel will look like
. After filling the atomic orbitals with electrons, taking into account the Hund's rule d-sublevel will look like ![]() .
.
Using now the concepts of the principles of least energy and Pauli, we distribute electrons in atoms according to energy levels (Table 1).
Table 1
The distribution of electrons over the energy levels of atoms
Using this scheme, it is possible to explain the formation of the electronic structures of the atoms of the elements of the periodic system, written in the form of electronic formulas. The total number of electrons in an atom is determined by the atomic number of the element.
So, in the atoms of the elements of the first period, one s-orbital of the first energy level (Table 1). Since there are two electrons at this level, there are only two elements in the first period (1 H and 2 He), the electronic formulas of which are as follows: 1 H 1 s 1 and 2 Not 1 s 2 .
In atoms of elements of the second period, the first energy level is completely filled with electrons. will be successively filled with electrons s- And R-sublevels of the second energy level. Sum s- And R-electrons that filled this level is eight, so there are 8 elements in the second period (3 Li ... 10 ne).
In atoms of elements of the third period, the first and second energy levels are completely filled with electrons. will be filled in succession s- And R-sublevels of the third energy level. Sum s- And R-electrons that filled the third energy level is eight. Therefore, in the third period there are 8 elements (11 Na ... 18 Ar).
In the atoms of the elements of the fourth period, the first, second and third are filled 3 s 2 3R 6 energy levels. At the third energy level, the free remains d-sublevel (3 d). The filling of this sublevel with electrons from one to ten begins after it is filled with maximum electrons 4 s-sublevel. Further, the placement of electrons occurs on 4 R-sublevel. Amount 4 s-, 3d- and 4p-electrons is equal to eighteen, which corresponds to 18 elements of the fourth period (19 K ... 36 Kr).
Similarly, the formation of electronic structures of atoms of elements of the fifth period occurs with the only difference that s- And R- sublevels are on the fifth, and d- sublevel on the fourth energy levels. Since the sum is 5 s-, 4d- and 5 R-electrons is eighteen, then in the fifth period there are 18 elements (37 Rb ... 54 Xe).
There are 32 elements in the extra-large sixth period (55 Cs ... 86 Rn). This number corresponds to the sum of electrons by 6 s-, 4f-, 5d- and 6 R-sublevels. The sequence of filling the sublevels with electrons is as follows. First filled with electrons 6 s-sublevel. Then, contrary to the Klechkovsky series, it will be filled with one electron 5 d-sublevel. After that, 4 will be filled to the maximum. f-sublevel. Next, 5 will be filled d- and 6 R-sublevels. The previous energy levels are filled with electrons.
A similar phenomenon is observed during the formation of electronic structures of atoms of elements of the seventh period.
Thus, in order to write the electronic formula of an atom of an element, you need to know the following.
1. Ordinal number of the element in the periodic system of elements D.I. Mendeleev, corresponding to the total number of electrons in an atom.
2. The number of the period, which determines the total number of energy levels in the atom. In this case, the number of the last energy level in the atom corresponds to the number of the period in which the element is located. In atoms of elements of the second and third periods, the filling of the last energy level with electrons occurs in the following sequence: ns 1–2 …nr 1–6. In atoms of elements of the third and fourth periods, the sublevels of the last and penultimate energy levels are filled with electrons as follows: ns 1–2 …(n–1)d 1–10 …nr 1–6. In the atoms of the elements of the sixth and seventh periods, the sequence of filling sublevels with electrons is as follows: ns 1–2 …(n–1)d 1 …(n-2)f 1–14 …(n–1)d 2–10 …nr 1–6 .
3. In the atoms of the elements of the main subgroups, the sum s- And R-electrons at the last energy level is equal to the group number.
4. In atoms of elements of secondary subgroups, the sum d-electrons on the penultimate and s-electrons at the last energy levels is equal to the group number, except for the atoms of the elements of the cobalt, nickel, copper and zinc subgroups.
The placement of electrons in atomic orbitals of the same energy sublevel occurs in accordance with Gund's rule: the total value of the spin of electrons located at the same sublevel should be maximum, i.e. this sublevel for each orbital first accepts one electron with parallel spins, and then - the second electron with the opposite spin.
Example 2 . Write the electronic formulas of the atoms of elements that have serial numbers 4, 13, 22.
Solution. The element with atomic number 4 is beryllium. Therefore, there are 4 electrons in a beryllium atom. Beryllium is in the second period, in the second group of the main subgroup. The period number corresponds to the number of energy levels, i.e. two. These energy levels must accommodate four electrons. The first energy level has two electrons (1 s 2) and the second also has two electrons (2 s 2) (see Table 1). Thus, the electronic formula has the following form: 4 Be 1 s 2 2s 2. The number of electrons in the last energy level corresponds to the number of the group in which it is located.
The element aluminum corresponds to the element 13 in the periodic system. Aluminum is in the third period, in the third group, in the main subgroup. Therefore, there must be three electrons in the third energy level, which will be placed in this way: 3 s 2 3R 1 (sum s- And R-electrons is equal to the group number). Ten electrons are in the first and second energy levels: 1 s 2 2s 2 2p 6 (see Table 1). In general, the electronic formula of aluminum is as follows: 13 Al 1 s 2 2s 2 2p 6 3s 2 3p 1 .
In the periodic system, the element with the atomic number 22 is titanium. There are twenty-two electrons in a titanium atom. They are placed on four energy levels, since the element is in the fourth period. When placing electrons in sublevels, it must be taken into account that this is an element of the fourth group of the side subgroup. Therefore, at the fourth energy level, s-there are two electrons in the sublevel: 4 s 2. First, second, third levels s- And R- sublevels are completely filled with electrons 1 s 2 2s 2 2p 6 3s 2 3p 6 (see Table 1). The remaining two electrons will be located on d- sublevel of the third energy level: 3 d 2. In general, the electronic formula of titanium is: 22 Ti 1 s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 .
"Slip" of electrons
When writing electronic formulas, one should take into account the "leakage" of electrons from s- sublevel of the external energy level ns on d- sublevel of the preexternal level ( n – 1)d. It is assumed that such a state is the most energetically favorable. "Slippage" of an electron occurs in the atoms of some d-elements, for example, 24 Cr, 29 Cu, 42 Mo, 47 Ag, 79 Au, 41 Nb, 44 Ru, 45 Rh, 46 Pd.
Example 3. Write the electronic formula of the chromium atom, taking into account the "breakthrough" of one electron.
Solution. The electronic formula of chromium, according to the principle of minimum energy, should be: 24 Cr 1 s 2 2s 2 2p 6 3s 2 3p 6 3d 4 4s 2. However, in the atom of this element, there is a "slip" of one s-electron from external 4 s- sublevel to sublevel 3 d. Therefore, the arrangement of electrons in a chromium atom is: 24 Cr 1 s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1 .
Practical work
1. Basic provisions
Periodic system of chemical elements and the structure of the atom
Modern definition of the Periodic Law
The properties of chemical elements and the substances they form are in a periodic dependence on the charges of their atomic nuclei
The table of the Periodic System of Chemical Elements is a graphical representation of the Periodic Law.
Each number in it characterizes some feature in the standing of atoms:
A)ordinal The (atomic) number of a chemical element indicates the charge of its atomic nucleus, that is, the number of protons contained in it, and since the atom is electrically neutral, the number of electrons around the atomic nucleus.
Number of neutrons determined by the formula:N = A - Z ,
WhereA - mass number (atomic mass),Z - serial number of the element;
b) the number of the period corresponds to the number of energy levels (electronic layers) in the atoms of the elements of the given period;
c) the group number corresponds to the number of electrons at the outer level for the elements of the main subgroups and the maximum number of valence electrons for the elements of the secondary subgroups.
Changing the metallic and non-metallic properties of elements
in periods and groups
1. Within one period with an increase in the serial number, the metallic properties of the elements weaken, and the non-metallic properties increase, since:
1) the number ē grows at the outer level of atoms (it is equal to the number of the group);
2) the number of energy levels within the period does not change (it is equal to the number of the period);
3) the radius of atoms decreases.
2. Within the same group (major subgroup) with an increase in the serial number, the metallic properties of the elements increase, and the non-metallic ones weaken, since:
1) the number of electrons at the outer level of atoms is the same (it is equal to the group number);
2) the number of energy levels in atoms grows (it is equal to the number of the period);
3) the radius of atoms increases.
Evidence of the complexity of the structure of the atom
1. The Irish physicist Stoney introduced the concept of "electron" to denote particles (for example, the electrification of an ebonite stick), the appearance of static electricity on clothes.
2. Cathode rays - the flow of electrons from the atoms of the metal from which the cathode is made caused the glass to glow (Thomson and Perrin). The negative charge of the electron was established. This smallest charge is taken as unity = -1.
Thomson also established its mass, equal to 1/1840 of the mass of a hydrogen atom.
3. Radioactivity is a phenomenon discovered by A. Becquerel. There are 3 types of radioactive rays:
a) α - rays, consisting of α - particles with charge +2 and mass 4;
b) β - rays - electron flow; c) γ - rays - electromagnetic waves.
Therefore, the atom is divisible and has a complex structure.
Table 1Planetary model of the atom (Rutherford)
CoreEqual to the number of nucleons (sum of protons and neutrons)
1) p + (have mass = 1 and charge = +1)
Their number is equal to the element number;
2) n 0 (have mass = 1 and charge = 0)
Their numberN = A r – Z. ( Zis the number of protons)
Electronic shell
Made up of electrons
(mass tends to zero and charge = -1);
Their number is equal to the element number.
All the mass of an atom is concentrated in the nucleus
The atom is electrically neutral
Atom - electrically neutral system of interacting elementary particles, consisting of a nucleus (formed by protons and neutrons) and electrons
The structure of the electron shells of atoms
The concept of the electron shell of the atom and energy levels
1. Electronic shell – the collection of electrons that surround an atomic nucleus.
2. In the electron shell, layers are distinguished on which electrons with different energy reserves are located, they are calledenergy levels . The number of these levels is equal to the number of the period in the periodic table.
3. The space around the nucleus, in which the electron is most likely to be found (about 90%), is calledorbital .
The size and shape of the orbitals
Rice. 1 Shapes of s-, p- and d-orbitals1) s 2 - electrons; spherical, symmetrical with respect to the nucleus and has no direction.
2) p 6 – electrons; dumbbell-shaped, located in the atom mutually perpendicular
There are orbitals of a more complex shape:d 10 - orbitals andf 14 - orbitals.
The number of energy levels (electronic layers) in an atom is equal to the period number in the D.I. Mendeleev, to which the chemical element belongs: the atoms of the elements of the first period have one energy level, the second period - two, the third period - three, the seventh period - seven.
The largest number of electrons in the energy level is determined by the formula:
N = 2 n 2 , WhereN- maximum number of electrons;
n- level number or main quantum number. (Integern, denoting the energy level number, is calledprincipal quantum number ).
Energy levels and electronic configuration of the atom
The atom has a complex structure. It consists of a nucleus, which includes protons and neutrons, and electrons revolving around the nucleus of an atom. The charge of the proton is +1, and the mass is 1 c.u. Neutron is an electrically neutral particle, the mass is approximately 1.e. Electron - charge is -1, mass 5.5∙10 -4 c.u. In general, the atom is electrically neutral, the number of protons in the nucleus of an atom is equal to the number of electrons in the atom. Electrons in an atom are distributed at energy levels.
The number of energy levels in an atom is determined by the number of the period in which the element is located. When building electronic models of atoms, it should be remembered that the maximum number of electrons at the energy level is 2n 2 , Wheren– energy level number. In accordance with this, at the first level closest to the nucleus there can be no more than 2 electrons, at the second - no more than 8, at the third - no more than 18, at the fourth - no more than 32. At the outer energy level there can not be more than 8 electrons.
Atomic absorption and emission spectra clearly show that all atoms have a number of possible energy states, called the ground and excited electronic states (Fig. 1).
Recording the distribution of electrons in an atom over electronic levels and sublevels is called its electronic configuration and can be made for both the ground and excited states of the atom. To determine the specific electronic configuration of an atom in the ground state, there are the following three provisions:
Filling principle (least energy). Electrons in the ground state fill the orbitals in a sequence of increasing orbital energy levels. The lowest energy orbitals are always filled first.
Pauli principle. No more than two electrons can be in any orbital, and with oppositely directed spins (spin is a special property of an electron that has no analogues in the macrocosm, which can be simplified as the rotation of an electron around its own axis).
Gund's rule. Degenerate (with the same energy) orbitals are filled with single electrons with the same spins, only after that the degenerate orbitals are filled with electrons with opposite spins according to the Pauli principle.
quantum numbers
Principal quantum number n equivalent to the quantum number in Bohr's theory. It basically determines the energy of electrons in a given orbital.
.....
....
Orbital quantum number l determines the value of the orbital angular momentum of the electron's momentum in a given orbital. Valid values: 0, 1, 2, 3, ... , n-1.
This quantum number describes the behavior of an atomic orbital during rotations of the coordinate system centered on the atomic nucleus.
Orbital magnetic quantum number m l determines the value of the component of the projection of the angular momentum of the electron on the selected direction in space. In the absence of an external magnetic field, electrons in orbitals with the same value of the orbital quantum numberl are energetically equivalent (i.e. their energy levels are degenerate).
However, in a constant magnetic field, some spectral lines split. This means that the electrons become energetically unequal. For example, p-states in a magnetic field take 3 values instead of one, d-states take 5 values. Allowed values m l for thisl : - l , ... -2, -1, 0, +1, +2, ... + l
Spin quantum number m s due to the presence of an intrinsic magnetic moment of the electron. In general, the expression for the magnetic moment of momentum coincides with that for the orbital moment:
For an electron m s takes only two values: +1/2 and -1/2. Sometimes, for a more visual explanation of the concept of spin, a rough analogy is used - an electron is represented as a flying top (a circular current that creates its own magnetic field). This analogy makes it possible to explain the presence of spin 1/2 for an electron and a proton, but not for a neutron, are particles with zero charge.
The concept of "spin" does not fit into our "macro-representations" of space. With all methods of its registration, the spin is always directed along the axis that the observer has chosen as the original one. A spin value of 1/2 means that an electron (proton, neutron) becomes identical to itself at a rotation of 720 0 , not 360 0 like in our 3D world. Spin is considered to be one of the fundamental properties of nature (i.e., it is non-derivable, like gravity and electricity).
Each orbital is indicated by a square cell, electrons by oppositely directed arrows (see the solution of exercises on this topic)
Electronic formula is a formula that shows the distribution of electrons on the electron layers in an atom.
table 2
Principal quantum number, types and number of orbitals, maximum number of electrons at sublevels and levels
Energy level(period number)
n
The number of sublevels equal to n
Shape (type) of orbitals
Number of orbitals
Maximum number of electrons
in sublevel
at a level equal to n 2
at sublevels
at levels
TO (n=1)
1 s
Practical work
Goal of the work:
6) Conclusion
Task number 1
5. Number of electronsN ē6 . Chargeatom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
a) by group
b) by period
Task number 2
1) the electronic formula of an atom of an element, according to the number of electrons at the outer level, metallic and non-metallic character (if there are 1-3 electrons at the outer level, then the element is a metal, if more than 3, then the element is a non-metal;
2) the electronic structural formula of the valence shell of the element atom, the normal and excited state of the atom, the negative and positive oxidation states for p - elements (non-metals), the highest and lowest positive oxidation states for metals ( s - And d - families);
3) the formula of a hydrogen compound (for s -element hydride with H - , For p - element gaseous hydrogen compound with H + ), name;
4) formulas of oxides in which positive oxidation states are manifested, name, indicate the nature;
5) formulas of bases and acids corresponding to oxides, name; salt formulas, name.
Characteristic p - element S - sulfur, located in III period of the main subgroup VI groups
1) 16 S 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 - non-metal, since at the outer level the atom has more than three electrons - six
2) S 3 s 2 3 p 4 p - element
↓ the normal state of an atom is 2 unpaired electrons, therefore,Ssulfur
S ↓ 3p 4 exhibits a negative oxidation state (-2):
3 s 2 S 0 + 2 ē →S -2
S * the first excited state is 4 unpaired electrons, therefore,S
3 d 1 exhibits a positive oxidation state (+4):
↓ 3 p 3 S 0 - 4 ē →S +4
3 s 2
the second excited state is 6 unpaired electrons, therefore,
3 d 2 sulfur exhibits a positive oxidation state (+6):
S ** 3 p 3 S 0 - 6 ē →S +6
3 s 1
3) S -2 → H 2 S- hydrogen sulfide, the aqueous solution of which is hydrosulfide acid.
saltH 2 Scalled sulfides; (name) K 2 S- potassium sulfide.
4) S +4 → SO 2 (sulfur oxideIV) → acidH 2 SO 3 → salt:
TO 2 SO 3 and KNSO 3
5) S +6 → SO 3 (sulfur oxideVI) → acidH 2 SO 4 → salt: K 2 SO 4 and KNSO 4
Characteristic s - element Ca - calcium, is in the fourth period of the main subgroup of the second group
1) 20 Sa 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 Kcalcium is a metal, since at the outer level the atom has less than three electrons - 2 electrons
2) Sa 4 s 2 s- element; Sa 4s 2 - normal state of the atom - no unpaired electrons
Sa * the excited state of the atom is two unpaired electrons, therefore,
Sa 0 - 2 ē → Sa +2
4p 1 Ca - exhibits a positive oxidation state (+2);negative degree
4 s 1 metals do not oxidize
3) Sa +2 H 2 - - hydrogen connection; SaN 2 (calcium hydride)
4) Sa +2 → oxide CaO → base Ca(OH) 2 → salt: 1) CaCI 2 and SaonCI 2) CaSO 3 AndCa(HSO 3 ) 2
Task number 3
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
s-element
R- element
Conclusion:
Practical work
Option 1
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide and their character
Task number 2
Characteristics of an element by its position in the periodic system, indicate the valence possibilities of an atom of an element
Task number 3 Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt formulas
s-element
p-element
Conclusion:
Practical work
Option 2
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
Task number 2
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 3
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
Task number 2
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 4
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Goal of the work:
1) Learn to characterize the elements by their position in the periodic system
2) Apply knowledge about the structure of the atom when compiling the characteristics of atoms of chemical elements
3) Write down the electronic formula of the element
4) Determine the formula and nature of the higher oxide and hydroxide; its hydrogen compound
5) Give a comparative description with neighboring elements in the period and group
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
Task number 2
Characterizing an element by its position in the periodic system, indicate:
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 5
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Goal of the work:
1) Learn to characterize the elements by their position in the periodic system
2) Apply knowledge about the structure of the atom when compiling the characteristics of atoms of chemical elements
3) Write down the electronic formula of the element
4) Determine the formula and nature of the higher oxide and hydroxide; its hydrogen compound
5) Give a comparative description with neighboring elements in the period and group
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
(acids and salts - following the example of nitric and nitrous acids)
Task number 2
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 6
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Goal of the work:
1) Learn to characterize the elements by their position in the periodic system
2) Apply knowledge about the structure of the atom when compiling the characteristics of atoms of chemical elements
3) Write down the electronic formula of the element
4) Determine the formula and nature of the higher oxide and hydroxide; its hydrogen compound
5) Give a comparative description with neighboring elements in the period and group
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
(acids and salts - for exampleS)
Task number 2
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 7
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Goal of the work:
1) Learn to characterize the elements by their position in the periodic system
2) Apply knowledge about the structure of the atom when compiling the characteristics of atoms of chemical elements
3) Write down the electronic formula of the element
4) Determine the formula and nature of the higher oxide and hydroxide; its hydrogen compound
5) Give a comparative description with neighboring elements in the period and group
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
(acids and salts - for exampleS)
Task number 2
Characterizing an element by its position in the periodic system, indicate:
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Practical work
Option 8
Drawing up electronic formulas of atoms of elements and graphic schemes, filling them with electrons
Goal of the work:
1) Learn to characterize the elements by their position in the periodic system
2) Apply knowledge about the structure of the atom when compiling the characteristics of atoms of chemical elements
3) Write down the electronic formula of the element
4) Determine the formula and nature of the higher oxide and hydroxide; its hydrogen compound
5) Give a comparative description with neighboring elements in the period and group
Progress
Task number 1
Fill in the table:
5. Number of electronsN ē6 . Charge atom nuclei, Z
7. Mass number, A
8. Number of neutrons,N n 0 = A -N R +
9. Write the distribution of electrons by energy levels
10. Comparison with elements neighbors:
a) by group
b) by period
11. Formula of higher oxide and hydroxide
(acid - boric, salts - borates)
Task number 2
Characterizing an element by its position in the periodic system, indicate:
Enter the results of the work in the table in the form:
Form ēelement
Element
Valence
shell
Lowest oxidation state
Hydrogen compound
Intermediate oxidation states
Highest oxidation state
Supreme Oxide Formula
Hydroxide Formula
Salt Formula
Conclusion:
Popular
- Celebration of the day of agriculture and processing industry
- When is Agriculture Day celebrated?
- Card games at the table
- Funny and funny contests for a fun company of adults
- Polish "paratroopers" for the Soviet marines
- Project 205 missile boats
- How is life on the new Chinese destroyer
- The newest frigate "Admiral of the Fleet Kasatonov" is preparing for the first tests and going to sea Ship Admiral Kasatonov
- Submarines of the Gato type
- Insignia on the merchant fleet of the USSR Detachment of the II group